Have you ever wondered why adding air to a tire increases its pressure? It’s a simple process, but the science behind it might surprise you. When you fill a tire with air, the pressure inside the tire increases because more air molecules are trapped inside. As a result, the tire becomes more firm and less prone to punctures, providing better stability for your vehicle.
This might seem obvious, but the relationship between air and pressure isn’t always intuitive. When you understand how air molecules behave and interact with each other, it becomes clear why adding air can make such a big difference. Let’s explore the science behind it.
Table of Contents
Pressure and Temperature Relationship
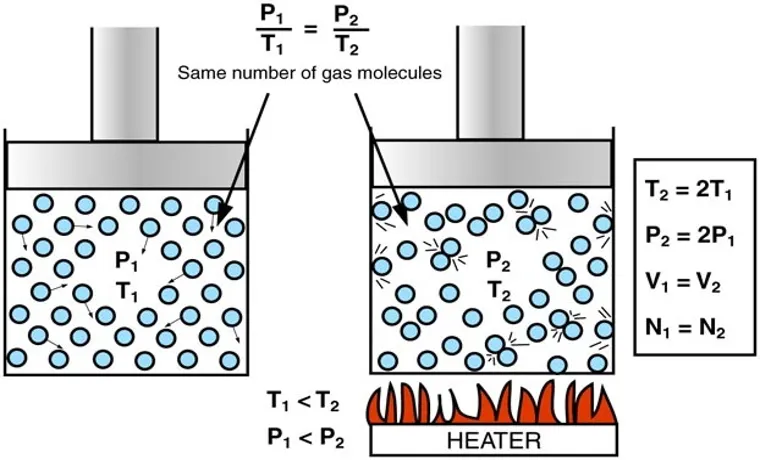
Have you ever wondered why pumping air into a tire at a constant temperature increases the pressure? The pressure and temperature of a gas are directly proportional to each other, meaning that as the temperature of a gas increases, so does its pressure. This is because an increase in temperature leads to an increase in the kinetic energy of the gas molecules, causing them to collide with the walls of the tire more frequently and with greater force. When you pump air into a tire, you compress the gas inside the tire, which increases its pressure.
As a result, the tire becomes harder and can support more weight. It’s important to note that this relationship between pressure and temperature only holds true under constant volume. In other words, if you were to pump air into a tire while heating it up, the tire may burst due to the increased pressure.
So next time you fill up your tire, you can thank the ideal gas law for keeping you and your car safe on the road.
Boyle’s Law
Boyle’s Law is a gas law that states that at a constant temperature, the pressure and volume of a gas have an inverse relationship. In simpler terms, if you increase the pressure of a gas, the volume will decrease, and vice versa, provided the temperature remains the same. This is because the molecules in a gas are constantly moving and colliding.
When the pressure is increased, the molecules are forced closer together, and there is less space between them, resulting in a smaller volume. Conversely, when the pressure is reduced, the molecules move farther apart, creating more space and leading to a larger volume. This law has many practical applications, including in the design of combustion engines and scuba diving equipment.
So, the next time you’re out for a dive or driving a car, remember the inverse pressure and volume relationship described in Boyle’s Law!
Charles’s Law
Charles’s Law is a gas law that describes the relationship between the pressure and temperature of a gas. This law states that when the temperature of a gas increases, the pressure also increases, assuming that the volume remains constant. Likewise, if the temperature decreases, the pressure of the gas also decreases.
This idea can be explained by imagining a balloon. When you blow up a balloon, the pressure inside the balloon increases, causing it to expand. However, if you cool the balloon by placing it in the fridge, the pressure decreases, causing it to shrink.
Charles’s Law is important in understanding the behavior of gases in different temperature environments. By studying this law, scientists and engineers can predict how gases will behave under different conditions and can use this information to develop more efficient and effective systems.
Gay-Lussac’s Law
Gay-Lussac’s law is a gas law that describes the relationship between pressure and temperature of a gas at a constant volume. This law states that the pressure of a gas is directly proportional to its absolute temperature when the volume is kept constant. In simpler terms, if the temperature of a gas increases, the pressure also increases, and if the temperature decreases, the pressure also decreases.
It is essential to note that this law applies only when the volume of the gas is constant, and the number of gas molecules is constant. For example, if you have a helium-filled balloon and place it in the refrigerator, the temperature of the helium decreases, and the pressure also decreases, causing it to deflate slightly. On the other hand, if you heat the balloon, the pressure increases, making it expand.
Gas Behavior with Temperature and Pressure
Have you ever wondered why pumping air into a tire at a constant temperature increases the pressure? Well, it is due to gas behavior with temperature and pressure. When air is pumped into a tire, the gas molecules inside get compressed and become more densely packed together. This increase in pressure causes the gas molecules to collide with each other more frequently, resulting in a higher temperature.
The ideal gas law states that pressure and temperature are directly proportional, meaning that as pressure increases, so does temperature. Therefore, as the pressure inside the tire increases due to pumping air, the temperature also increases. This gas behavior is essential in everyday life, from the air we breathe to the gases used in industry.
Understanding the relationship between pressure and temperature of gases can help us control and predict their behavior in various situations.
Ideal Gas Law
Gas behavior, Ideal Gas Law, Temperature, Pressure Gas behavior is fascinating and complex, yet it can be understood by considering the Ideal Gas Law. This law summarizes the relationship between the behavior of gases, temperature and pressure. The law states that the pressure, volume, and temperature of a gas are interdependent.
It implies that with an increase in temperature, the gas molecules move faster and more vigorously, leading to an increase in pressure. Similarly, a decrease in volume results in a rise in pressure. At constant pressure, a rise in temperature leads to an increase in volume, while a decrease in temperature leads to a decrease in volume.
It is incredible to see how small variations in temperature and pressure can affect the behavior of gases in various environments. The Ideal Gas Law is a fundamental concept in thermodynamics and plays a crucial role in understanding gas dynamics in industries, medicine, and the environment.
Molecular Theory
As we all know, gas is a vital aspect of our daily lives. From providing electricity to powering vehicles, gases play an indispensable role. But have you ever wondered how gas behaves under different temperature and pressure conditions? Well, the molecular theory explains this phenomenon.
When gas particles are heated, they move faster, which increases their kinetic energy. This increase in energy causes the gas to expand, resulting in an increase in pressure. Similarly, when the gas is compressed, the particles move closer together, reducing the volume, and thereby increasing the pressure.
This can be seen in the example of a balloon that expands as we put hot air in it, while it contracts when cool air is added. Molecular theory is essential in understanding gas behavior and helps us predict how gases will act under different conditions.
Effect of Pumping Air at a Constant Temperature
Have you ever wondered why pumping air into a tire at a constant temperature increases the tire pressure? It all boils down to the ideal gas law. When air is pumped into a tire, the number of gas molecules inside the tire increases, which means that the pressure inside the tire increases as well. The ideal gas law states that the pressure of a gas is directly proportional to the number of molecules of gas present in the container, as long as the temperature of the gas stays constant.
This means that the more gas molecules there are inside the tire, the higher the pressure will be. So, when you pump air into a tire, you are increasing the number of gas molecules inside the tire, and thus, increasing the pressure. It’s important to note that this only holds true if the temperature stays constant.
If the temperature changes, the pressure will also change, as the volume will vary accordingly. Overall, pumping air into a tire is a simple but effective way to increase the pressure inside the tire, which in turn makes it safer to drive on the road.
Explanation of Pressure Increase
When air is pumped into a container, the pressure inside the container increases. This is because the air molecules are compressed into a smaller space, leading to more collisions between the molecules and the walls of the container. The temperature of the air does not change during this process, which means that the pressure increase is solely due to the compression of the air.
Think of it like a crowded elevator – if you add more people into the same amount of space, the pressure inside the elevator increases. The same concept applies to pumping air into a container – as more air is added, the pressure inside the container increases. This increase in pressure has a wide range of practical applications, from inflating tires to powering tools and machinery.
However, it is important to be cautious when working with compressed air, as it can be potentially dangerous if not handled properly.
Conclusion
In the words of Archimedes, “Give me a lever long enough and a place to stand, and I will move the world.” Well, in the case of pumping air into a tire, the lever is the air molecules and the place to stand is the temperature. As we pump air into the tire, we are compressing those little molecules, giving them less space to move around.
And since temperature is all about the movement of molecules, if we keep that temperature constant, those compressed molecules will start bouncing around even more, creating more pressure in the tire. So although we may not be moving the world, we certainly are moving those air molecules!
FAQs
How does increasing the amount of air in a tire affect the pressure?
Increasing the amount of air in a tire increases the pressure inside the tire.
Does the temperature affect the pressure in a tire when you add air?
Yes, if the temperature of the tire remains constant, adding air will increase the pressure. However, if the temperature changes, so will the pressure.
What happens if you overinflate a tire?
Overinflating a tire can cause it to burst, because of too much pressure built up inside.
Can a decrease in air pressure affect a vehicle’s fuel efficiency?
Yes, a decrease in air pressure can cause a decrease in fuel efficiency due to the increased friction and resistance on the tires.
How often should you check the air pressure in your tires?
It’s recommended to check the air pressure in your tires once a month or before embarking on a long drive.
What type of pump should you use to fill up your tires?
It’s best to use a high-quality tire inflator that is designed specifically for tire pressure.
How does tire pressure affect a vehicle’s handling and braking?
Proper tire pressure is important for safe handling and braking. Underinflated tires can cause poor handling and braking performance, while overinflated tires can reduce traction and cause the vehicle to skid.